Windhoek, Namibia. August 2015 - August 2018.
In an ongoing project funded by the Center for Disease Control under the direction of the University of Namibia School of Pharmacy, we are transforming written UNAM curriculum into open-source digital materials.
ARV: Stavudine is a series that is going to be available for public use. As counterfeit HIV medicine cases increase, there is a recognized need that all pharmacists in Namibia should be equipped with the knowledge of how to test the authenticity of the drugs prior to patient administration. Our series will be published through the online KuraCloud campus and will be made available to the public.
“A. is for Aspirin,” is a video series that was translated from a written pharmacy lab. It is already worked into the first-year Pharmacy School curriculum, and student evaluations are completed. We have investigated the learning effectiveness in a submitted publication to the Journal of Chemical Education, status pending.
This page is just previewing the materials. The University of Namibia is finalizing the publication with KuraCloud for final online presentation.
All graphics by Emily Julka.
“ARV: Stavudine: Verification of Drug Identity and Content via Thin Layer Chromatography,”
General Publication. University of Namibia, 2018.
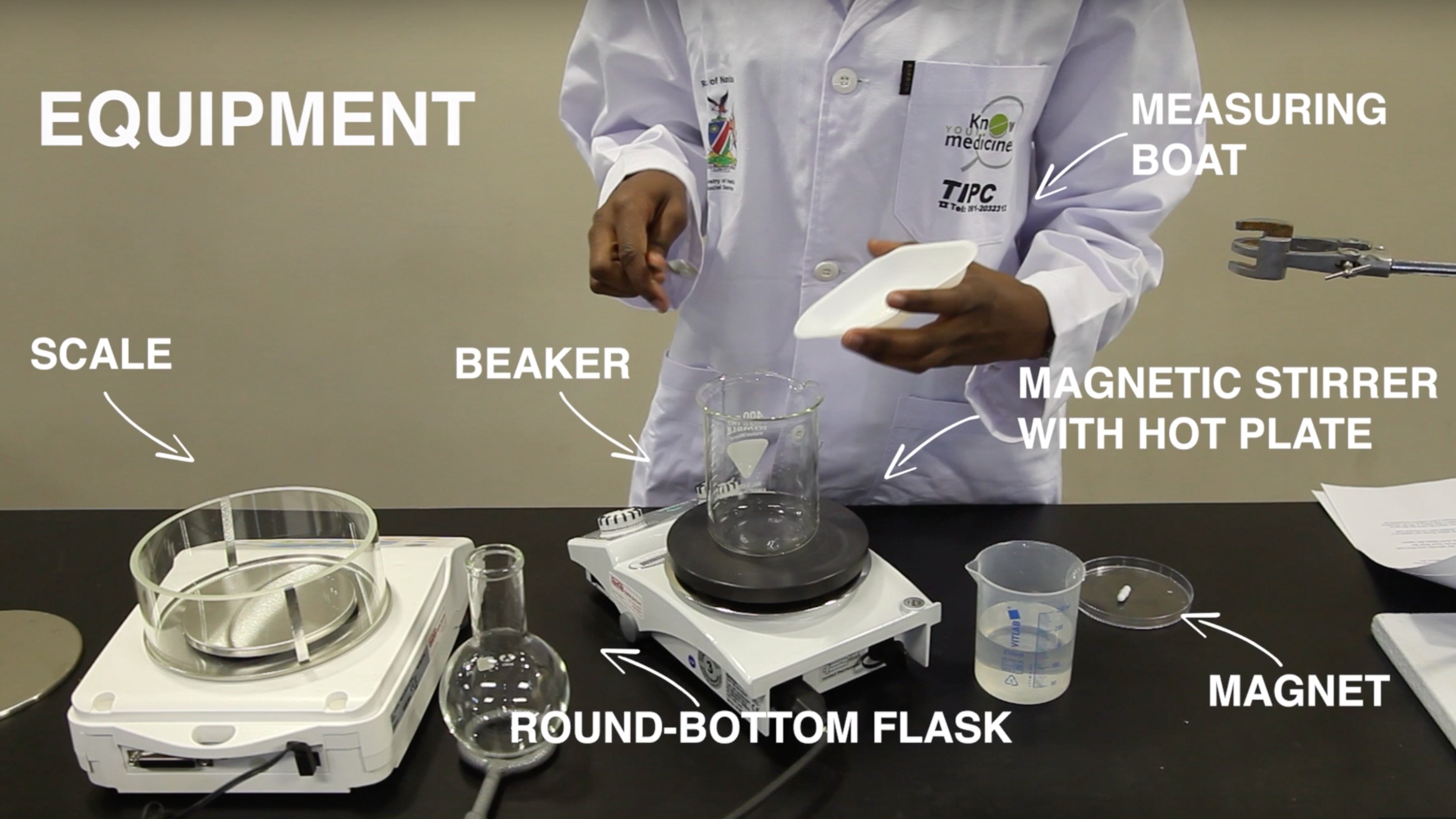
This lab is broken into 9 parts - A, B, C, D, E, F, G, H, I. Video names correspond with the letters written in the lab process graphics below. These are meant to work in conjunction with the written lab.
In parts A and B, you are using an authentic stavudine tablet to prepare a standard stock, and from that preparing two working stocks that will be standard solutions to indicate drug quality.
A. Preparing Stock
The preparation of the stock standard solution requires an authentic drug product for reference purposes, for example, capsules containing 40mg of stavudine. Open one capsule by carefully separating the cap from the bottom shell and transfer all the powder into a 25-ml laboratory glass bottle .adding the empty capsule shells last. For extraction, suspend the powder obtained in 16ml of water using a straight pipette. Close the bottle and shake for about three minutes until most of the solids are dissolved. Allow the solution to sit for an additional five minutes until undissolved residues settle below to the supernatant liquid. The solution obtained should contain 2.5mg of total drug per ml and be labelled as ‘Stavudine Stock Standard Solution’. Freshly prepare this solution for each test. Continue to work with the clear or hazy supernatant liquid.
B. Preparation of the Working Standard Solution 100% (Upper Working Limit)
Pipette 2ml of the stock standard solution into a 10-ml vial and add 2ml of methanol. Close and shake the vial. The solution obtained should contain 1.25 mg of total drug per ml and be labelled as "Stavudine Working Standard Solution 100%”.
This higher working standard solution represents a drug product of good quality containing 100% stavudine.
Preparation of the Working Standard Solution 80% (Lower Working Limit)
Pipette 2ml of the stock standard solution into a 10-ml vial and add 3ml of methanol. Close and shake the vial. The solution obtained should contain 1.0mg of total drug per ml and be labelled as “Stavudine Working Standard Solution 80%”.
This lower working standard represents a drug product of poor quality containing just 80% of the amount of stavudine as stated on the product’s label. In the current investigation, this drug level represents the lower acceptable limit for a given product.
In parts C and D, you are taking a sample stavudine tablet(s) to prepare a sample stock, and from that preparing a working sample solution that will be tested for drug quality.
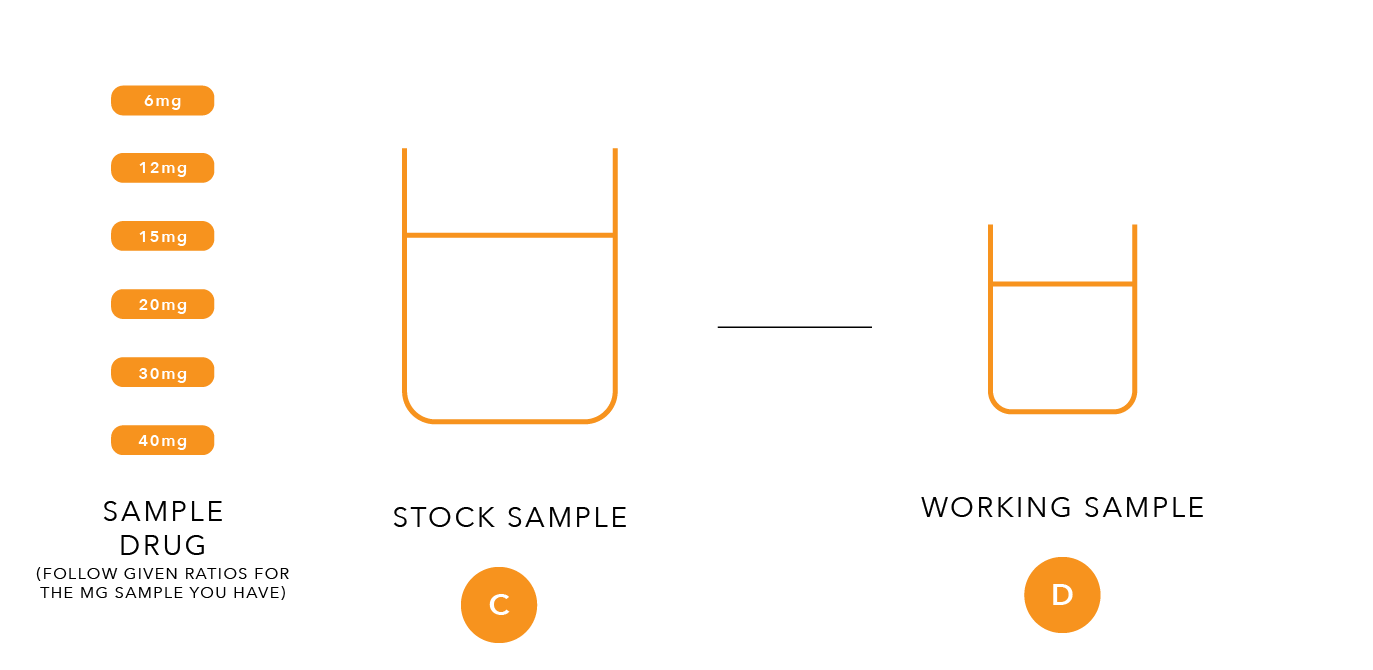
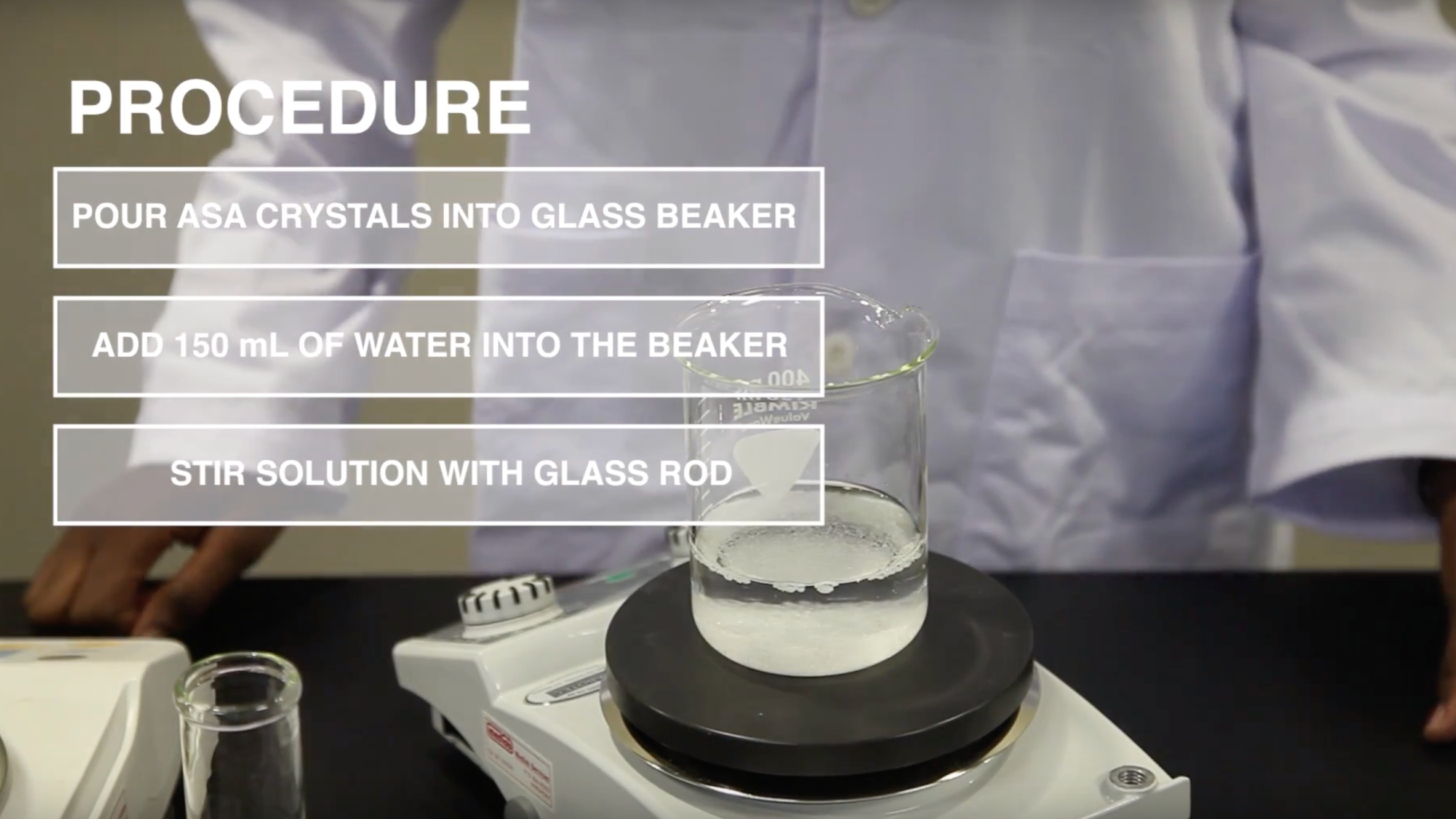
C. Preparation of the Stock Sample Solution from solid dosage forms claiming to contain 20mg of Stavudine per unit.
Take two whole tablets or capsules from an appropriate drug product sampled in the field. Powder obtained from sample capsules should be transferred directly into the bottle adding the cap and body shells last. For extraction, add 16ml of water using a straight pipette, close the bottle and shake for about three minutes until most of the solids are dissolved. Allow the solution to sit for an additional five minutes until undissolved residues settle below the supernatant liquid.
D. Preparation of the Working Sample solution
Pipette 2ml of the stock sample solution into a 10-ml vial and add 2ml of methanol. Close and shake the vial and label as “Stavudine Working Sample Solution”. The expected concentration of stavudine in this working sample solution is 1.25 mg per ml and should match the concentration of stavudine of the higher working standard solution produced above.
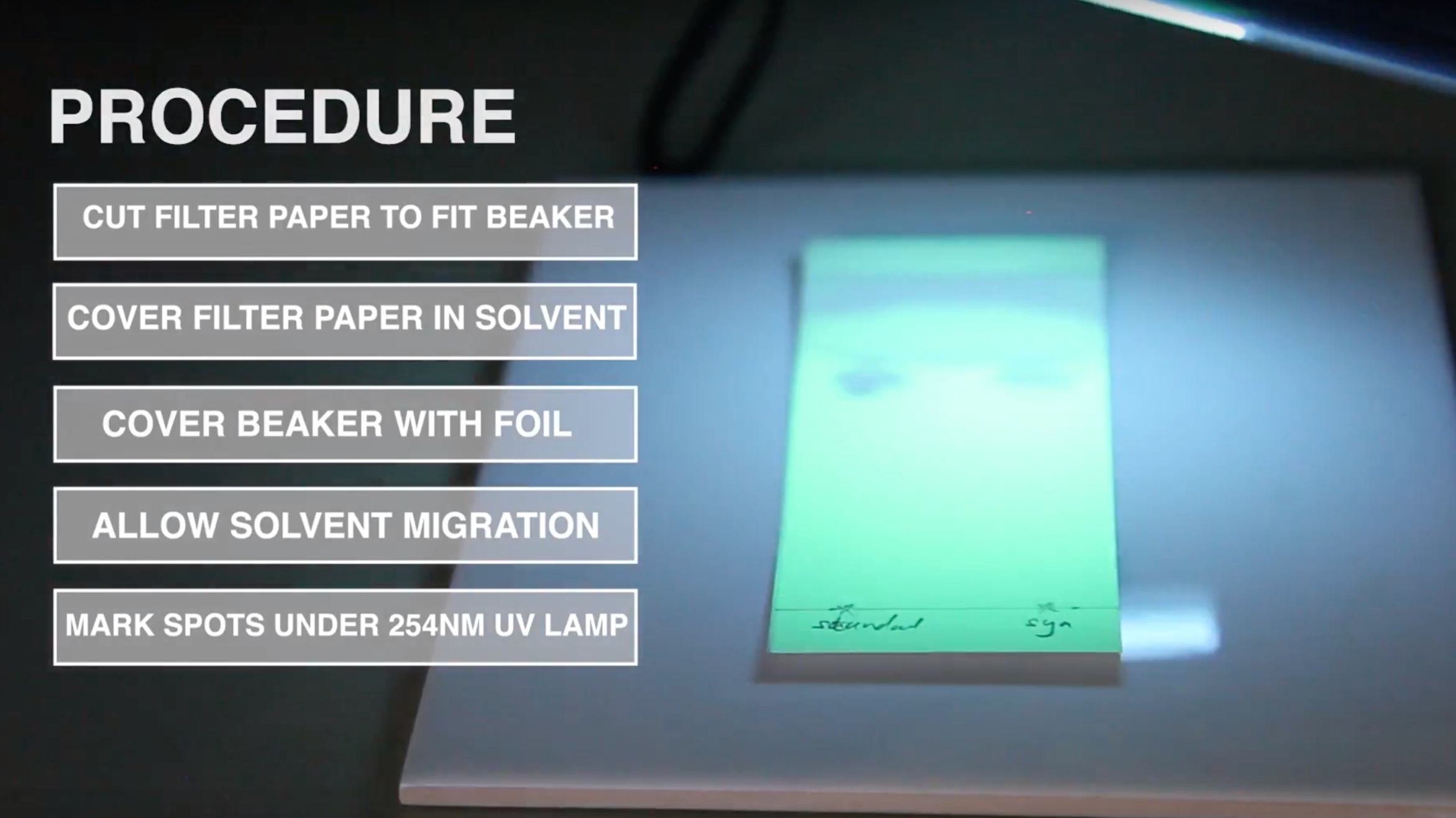
E. Development
Pipette 2ml of ethyl acetate, 10ml of methanol, and 8ml of toluene into the jar being used as TLC developing chamber. Close the chamber and mix thoroughly. Line the chamber’s wall with filter paper and wait for about 15 minutes thus ensuring saturation of the chamber with solvent vapour.
F. Spotting
Mark an origin line parallel to and about 1.5cm from the bottom edge of the chomatoplate and apply 2 µl of each test and standard solution as shown in the picture opposite using the microcapillary pipettes supplied.
Up to five spots can be placed on a plate. Check the uniformity of all spots using UV light of 254nm. All spots should be circular in shape and equally spaced across the origin line. Although their intensities might differ, their diameters never should. Different intensities are due to residual amounts of tablet and capsule excipients or different drug concentrations in the sample solutions. A difference in spot size, however, relates to poor spotting. Repeat this step if homogeneous spotting is not achieved the first time.
Note that the filling of the microcapillary pipettes might take some time when handling aqueous sample solutions. As traces of water are causing spot tailing, completely dry off all extraction solvent before chromatoplate development using the hot plate supplied.